Cell Therapy: A View Towards Advantages and Disadvantages of Four Approaches
Immune effector cell (IEC) therapies, commonly referred as cell therapies, utilize human cells to cause or enhance an immune response against tumor cells. Following remarkable successes with several patients in the early 2010s – most notably, the case of Emily Whitehead, the first pediatric CAR-T patient, who recently celebrated 10 years of being cancer-free – IECs have become a key focus of investigations to help treat multiple types of cancer, resulting in several approved therapies and hundreds of ongoing trials.
Here, we briefly define the four main groups of IECs currently under research: chimeric antigen receptor T cells (CAR-T), engineered T cell receptor (TCR) T cells, tumor-infiltrating lymphocytes (TILs), and natural killer (NK) cells, with a view towards advantages and disadvantages of each approach.
CAR-Ts: the first and currently only approved IEC therapy, autologous CAR-Ts take advantage of the patient’s own T cells to fight the tumor. Circulating T cells are collected and engineered ex vivo to express a customized receptor, the CAR, which recognizes and binds to a surface protein (antigen) in the tumor cell, inducing cell death.
- Advantages: can target any protein expressed in the tumor cell surface; do not rely on antigen presentation by human leukocyte antigen (HLA) complex; capable of memory formation and long-term persistence to monitor and prevent tumor recurrence; approved by the FDA for certain patient populations with hematological tumors
- Disadvantages: limited to proteins expressed in the cell surface; do not distinguish tumor from non-tumor proteins, leading to high risk of on-target, off-tumor toxicity; vulnerable to relapse due to antigen escape (loss or downregulation of target surface protein); autologous CAR-Ts involve a complex manufacturing process that require several weeks from prescription to infusion and are very expensive due to their personalized, non-scalable nature
CAR-Ts can also be generated from allogeneic sources (i.e., T cells collected from healthy donors rather than the patient’s own T cells), reducing costs and shortening time to treatment; however, allogeneic cells require additional precautions to prevent graft vs. host disease. Data for allogeneic CAR-Ts remains in the early stages, and no allogeneic CAR-Ts have been approved so far.
TCR Ts: as in autologous CAR-Ts, T cells are collected and engineered ex vivo, in this case, to express engineered TCRs. The chosen receptor usually derives from a library of endogenous TCRs that are known to target tumor-associated antigens.
- Advantages: potential to recognize any antigen that would be presented by HLA complex (i.e., not restricted to cell surface proteins); “natural” signaling (as opposed to CAR-Ts) may translate to reduced toxicity and ability to recognize and target low-density antigens
- Disadvantages: risk of mispairing endogenous and inserted TCR subunits could lead to off-target toxicity; patient population for any given TCR is limited by HLA subtype; as with CAR-Ts, manufacturing is complex and time-consuming, and when approved, products are likely to be similarly expensive
TILs: unlike CAR- and TCR-Ts, TIL therapies are not engineered to target a specific antigen; instead, they are naturally occurring T cells with antitumor activity. TILs are collected from a solid tumor sample, separated from the tumor cells, expanded ex vivo, and tested for tumor recognition. The lymphocytes (T cells) that successfully target and kill tumor cells are delivered back to the patient.
- Advantages: long-term persistence of lymphocytes circulating within immune system; ability to target multiple antigens and increase efficacy against solid tumors, which are highly heterogeneous; next-generation TIL therapies are being engineered for enhanced activity, persistence, and resistance to immunosuppressive tumor microenvironment
- Disadvantages: require patients to have preexisting lymphocytes with high and specific antitumor activity in a tumor that is accessible for biopsy; allogeneic TILs are unlikely to be feasible, since TILs are selected based on endogenous activity against the tumor
NK cells: a rapidly emerging field in IEC, NK cells play a role in immunosurveillance and are guided to tumor sites by chemokines and their associated receptors. NK cells do not naturally express TCRs but could be engineered to express TCRs or CARs.
- Advantages: allogeneic NK cells do not cause graft vs. host disease (as allogeneic T cells may) and are unlikely to cause cytokine release syndrome; capable of antibody-dependent, cell-mediated cytotoxicity (ADCC: antibodies bind to surface antigens on the tumor cell, the NK cell recognizes the antibodies, and mediates cell lysis to destroy the tumor cell), which enables combination of NK cells with therapeutic antibodies to target additional antigens and enhance antitumor response; many sources for collection of NK cells (peripheral blood mononuclear cells, umbilical cord blood, hematopoietic progenitor cells, iPSCs); several studies currently exploring potential of allogeneic NK cells
- Disadvantages: autologous NK cells may not be feasible since they are usually dysfunctional in cancer patients; short term persistence, due to NK cells being part of innate immune system and general shorter persistence of allogeneic vs. autologous cells
There are now six autologous CAR-T therapies approved by the FDA, all of which target either CD19 (for ALL and non-Hodgkin’s lymphoma) or BCMA (for multiple myeloma). These therapies have demonstrated high and durable response rates in late-stage diseases, which are likely to be cures in some cases.
All these successes have come in hematologic malignancies, but even in those indications, less than 50% of patients achieve long-term remissions. Therefore, there is substantial room for improvement, and many next-generation approaches are under investigation, including multi-antigen targeting, resistance to immunosuppression, and checkpoint inhibition.
New approaches aim to both improve outcomes in hematologic malignancies and drive meaningful efficacy in solid tumors, which are more complex and difficult to treat. Furthermore, cost and time requirements will drive advances in allogeneic, off-the-shelf products and point-of-care manufacturing with the goal of expanding accessibility of these life-saving therapies.
To facilitate understanding and tracking of the complex and rapidly growing field of IEC therapies, SAI’s team of experts has developed CellTraQ, a comprehensive database of cell therapy assets and clinical trials. Whether you want to discuss our strategic consulting capabilities in Oncology or discover the power of CellTraQ, please contact us today.
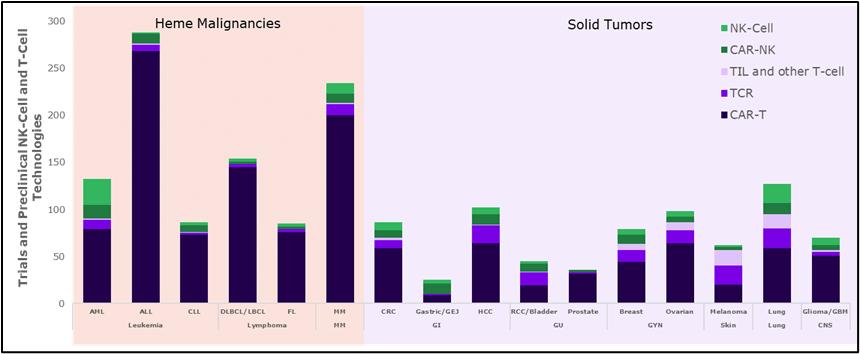
CellTraQ data search of oncology studies involving T and NK cells divided by tumor type.
Similar posts:
In the healthcare regulation sphere, proposed EU-level joint clinical assessments (JCAs) have sparked debate in the pharmaceutical industry. A recent EFPIA report highlights concerns about the rigidity of the guidelines, particularly in oncology, and suggests revisions to ensure a more efficient and adaptable evaluation process that accommodates diverse stakeholder needs across Europe. In the realm […]
A Comprehensive Exploration of Competitive Intelligence, Knowledge-Sharing, and Strategic Optimization in the Evolving Landscape of EU Health Technology Assessments. The first companies undergoing the JCA process in 2025 will indeed be pioneers, blazing a trail for others to follow. These early adopters will be the first to gain practical experience with the JCA, providing valuable […]
What rare disease day means for our work at SAI Every leap year, on the 29th of February, the world pauses to acknowledge Rare Disease Day—a beacon of solidarity for the millions navigating the labyrinth of rare and often isolating medical conditions. It’s a day that transcends boundaries, echoing the voices of patients, caregivers, and […]

